Abstract
The objective of the present study was to elucidate the effect of BMN 673 (talazoparib) on BRCA1 mutant (HCC1937) and wild-type (MDA-MB-231) triple negative breast cancer (TNBC). The in vitro cytotoxicity results indicated that BMN 673 had considerable inhibitory effects on HCC1937 and MDA-MB-231 cell lines by inducing apoptosis, multicaspase activity, G2/M arrest, and altering the expression levels of apoptosis-related genes (P less than 0.01). Additionally, BMN 673 indicated no toxicity on MCF-10A control cells until a certain concentration and incubation time. However, BMN 673, a novel and selective poly ADP ribose polymerase inhibitor, was more potent in TNBC cells bearing BRCA1 mutant than those with wild-type BRCA1. In conclusion, our study, for the first time, demonstrated a molecular mechanism of the induction of apoptosis by BMN 673 in TNBC with different genetic profile. However, further investigations regarding the exact molecular mechanisms underlying BMN 673-inducing apoptotic death and gene-cell line associations are required.
Key Words
Apoptotic death, BMN 673 (talazoparib), BRCA, triple negative breast cancer.
Abbreviations
dsDNAs, DNA double-strand breaks; HR, homologous recombination; PARP, poly ADP ribose polymerase; TN, triple negative; TNBC, triple negative breast cancer.
Introduction
Triple negative breast cancer (TNBC), accounts for 15% to 20% of all breast cancers, is defined by low or lack of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 expression. TNBC is characterized by aggressive clinical behavior, poor prognosis in the first 5 years and the lack of common therapeutic targets for effective therapy compared to other types of breast cancer.
The triple negative (TN) phenotype of breast cancer tumors is identified in patients with BRCA1/2 mutations or BRCAness phenotype. BRCA1 germline mutation is found in approximately 10% to 15% of TNBC patients and nearly 80% of hereditary BRCA1-mutated cancers are TNBCs. Furthermore, BRCAness phenotype, refers to sporadic TNBC, has shown considerable similarities with BRCA1-mutated TN tumors due to including BRCA1/2 dysfunctions and/or homologous recombination (HR) mechanism deficiency.
Poly ADP ribose polymerase (PARP) inhibitors have attracted a great deal of attention for the treatment of TNBC through the concept of synthetic lethality. PARP inhibition results in an increase DNA double-strand breaks (dsDNAs). Cells defective in HR cannot repair such toxic dsDNAs and thus, PARP inhibitors induce cell death in BRCA defective cancer cells. On the other hand, recent clinical and preclinical studies have focused on the efficacy and clinical utility of PARP inhibitors on TNBC patients with other alteration in HR mechanism outside of germline BRCA mutation.
BMN 673 (talazoparib) is considerably more potent than other PARP inhibitors at nanomolar concentrations and thus, has shown exciting results in preclinical studies and clinical trials for patients with advanced BRCA1 or BRCA2 hereditary and also sporadic breast and ovarian cancers. In the literature, BMN 673 has demonstrated in vitro and in vivo antitumor activity in the different cancer cell lines (ovarian cancer, lung cancer, pancreatic cancer, prostate cancer, osteosarcoma, and chronic lymphocytic leukemia). However, there is no study to assess BMN 673 efficacy in TNBC cell lines with different genetic backgrounds.
In this context, the aim of the present study was to investigate the efficacy of BMN 673, the most potent PARP inhibitor, for the treatment of TNBC with respect to the role of BRCA1 mutation status. Our results provide evidence for the first time that BMN 673 may have a therapeutic effect on TNBC regardless of BRCA1 mutation status.
Materials and Methods
Materials
BMN 673 (50 mg) was obtained from SelleckChem (Houston, TX). Dulbecco’s modified Eagle’s medium (DMEM), DMEM-F12, fetal bovine serum (FBS), penicillin-streptomycin, dimethyl sulfoxide and acridine orange (AO) were purchased from Sigma-Aldrich (St Louis, MO). Roswell Park Memorial Institute (RPMI) 1640 with L-glutamine was purchased from Lonza (Basel, Switzerland). The WST-1 Cell Proliferation Kit was purchased from Biovision (San Francisco, CA). The Muse Annexin V, Dead Cell Assay Kit, MultiCaspase and Cell Cycle Assay Kit were supplied by Merck Millipore (Darmstadt, Germany). The RNA isolation kit was purchased from Omega Bio-Tek. The High-Capacity cDNA Reverse Transcription Kit, Taqman primers and Taqman Master Mix were supplied by Thermo Fisher Scientific (Waltham, MA).
Cell Culture
The human triple negative breast carcinoma HCC1937 (BRCA1 5382insC mutation), MDA-MB-231 (BRCA1 normal) and MCF-10A normal human mammary epithelial cell lines were purchased from the American Type Culture Collection (ATCC, Rockville, MD). HCC1937 cells were cultured in RPMI medium, whereas MDA-MB-231 cells were maintained in DMEM medium supplemented with 10% FBS, penicillin and streptomycin (100 U/mL) at 37 degrees Celsius in a humidified atmosphere containing 5% CO2. Additionally, MCF-10A cells were grown in DMEM-F12 medium supplemented with 100 mg/mL EGF, 1 mg/mL hydrocortisone, 10 mg/mL insulin, 10% FBS, and penicillin and streptomycin (100 U/mL) at 37 degrees Celsius in a humidified atmosphere containing 5% CO2.
WST-1 Analysis
The viability of HCC1937, MDA-MB-231, and MCF-10A cells after exposure to different concentration of BMN 673 was analyzed by WST-1 assay. HCC1937 (2 × 10 to the power of 3/well), MDA-MB-231 (2 × 10 to the power of 3/well), and MCF-10A (1 × 10 to the power of 3/well) cells were seeded into in a 96-well culture plates due to differences in the growth rate. After a 24-hour incubation, these cell were treated with different concentrations (0.01, 0.05, 0.1, 0.5, 1, 5, and 10 nM) of BMN 673 and incubated for 6 to 12 days. Then, 10 microliters WST-1 dye (Biovision, San Francisco, CA) was added to each well and incubated for 1 to 3 hours at 37 degrees Celsius. Finally, the cell viability was measured in absorbance at 450 nm (Tecan, Männedorf, Switzerland). The number of cells in per well, exposure concentration and duration (6 to 12 days) were adjusted according to the literature.
Annexin V and Dead Cell Assay
To determine the apoptotic effects of BMN 673 on HCC1937, MDA-MB-231 and MCF-10A cells, Annexin V and Dead Cell kit (Millipore, Darmstadt, Germany) was used. After treatment with different concentrations (0.01, 0.1, 1, and 10 nM) of BMN 673 for 6 and 12 days, the cells were collected and washed with phosphate-buffered saline (PBS). Afterwards, 100 microliters MUSE annexin V and dead cell reagent was added to each tube and then, incubated for 30 minutes at room temperature in the dark. Finally, the cells were analyzed using a Muse Cell Analyzer (Muse EMD Millipore Co, Hayward, CA).
Cell Cycle Analysis
To evaluate cell-cycle arrest in HCC1937, MDA-MB-231, and MCF-10A cells, Muse Cell Cycle Assay Kit (Millipore, Darmstadt, Germany) was used. Briefly, the cells were grown in six-well plates and treated with 0.01, 0.1, 1, and 10 nM concentrations of BMN 673 for 6 and 12 days. After incubation, the cells were trypsinized, washed twice with PBS and fixed in 70% ethanol for 3 hours at 4 degrees Celsius. After fixation, the cells were centrifuged, washed with once with PBS and stained 200 microliters of Muse cell cycle reagent for 30 minutes at room temperature. Finally, the cells were analyzed by Muse Cell Analyzer (Muse EMD Millipore Co., Hayward, CA).
Multicaspase Analysis
To detect multiple caspases (caspase-1, 3, 4, 5, 6, 7, 8, and 9) activation in HCC1937, MDA-MB-231, and MCF-10A cells after treatment with BMN 673, Muse MultiCaspase Assay (Millipore, Darmstadt, Germany) was used according to manufacturer’s protocol. In brief, the cells were treated with 0.01, 0.1, 1, and 10 nM BMN 673 for 12 days and then centrifuged and washed with PBS. Then, cell suspensions were stained with 5 microliters of Muse MultiCaspase Reagent and incubated for 30 minutes in the 37 degrees Celsius incubator with 5% CO2. After incubation, 150 microliters of Muse Caspase 7-AAD working solution was added to each tube and analyzed by Muse Cell Analyzer (Muse EMD Millipore Co., Hayward, CA).
Quantitative Reverse-Transcription Polymerase Chain Reaction Analysis
Total RNA was extracted from the HCC1937, MDA-MB-231 and MCF-10A cell lines after 6 and 12 days of incubation with 0.1 and 10 nM BMN 673 using EZNA Cycle Pure Kit (Omega Bio-tek). The complementary DNA for messenger RNA (mRNA) expression was synthesized using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA). The relative mRNA expression of genes (Bax, Bcl-2, CCND1 [cyclin D], RB1, CDKN1A [p21], and housekeeping gene [ACTB]) involved in the regulation of apoptosis was performed on a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA) using Taqman Master Mix.
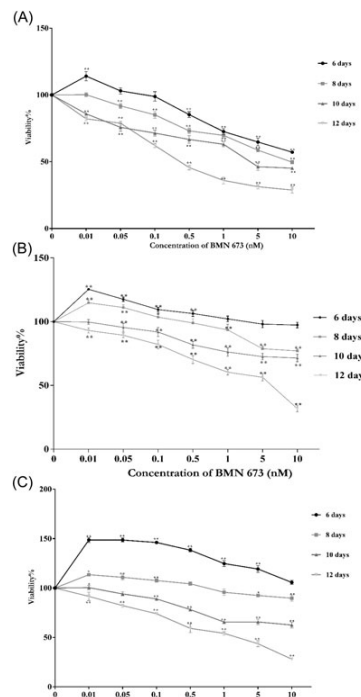
Figure 1: WST-1 Results WST-1 results of (A) HCC1937, (B) MDA-MB-231, and (C) MCF-10A cells treated with different concentration (0.01 to 10 nM) of BMN 673 for 6 to 12 days. The percentages compared with medium were expressed as means plus or minus SD and P less than 0.05 and P less than 0.01.
AO/Ethidium Bromide Double Staining
To observe morphological changes in BMN 673-treated HCC1937, MDA-MB-231, and MCF-10A cells, AO/ethidium bromide (Et-BR) double staining was performed. The cells were grown in six-well plates and treated with 0.01 and 10 nM concentration of BMN 673 for 12 days. Afterward, the cells were fixed in 4% paraformaldehyde (Merck, Germany) for 30 minutes. After fixation, the cells were washed three times with PBS and stained with AO/Et-BR solution (Sigma-Aldrich, St Louis, MO). Finally, the stained cells were visualized using an EVOS FL Cell Imaging System (Thermo Fisher Scientific, Waltham, MA).
Statistical Analysis
Statistical analysis of the data was performed with SPSS 22.0 (SPSS Inc, Chicago, IL). All the data were representative of three independent experiments. A one-way analysis of variance with the posthoc Tukey was used to compare multiple variables. P less than 0.05 was considered to be statistically significant difference (P less than 0.05 and P less than 0.01). Additionally, RT2 Profiler PCR Array Data Analysis was used for quantitative polymerase chain reaction (qPCR) data analysis.
Results
Cytotoxic Effects of BMN 673 on the Viability of TNBC
To analyze the cytotoxic effects of BMN 673 on the viability of HCC1937, MDA-MB-231, and MCF-10A cells, WST-1 analysis was used and the results were shown in Figure 1. As shown in Figure 1A and 1B, the viability of HCC1937 and MDA-MB-231 cells decreased to 57.2% (P less than 0.01) and 97.3% at 10 nM BMN 673 for 6 days, whereas the cell viability was 28.8% and 31.8% at a dose of 10 nM BMN 673 for 12 days, respectively (P less than 0.01). The MCF-10A cell viability was 105.7% at 10 nM BMN 673 for 6 days, while the cell viability was reduced to 28.1% at dose of 10 nM BMN 673 for 12 days (P less than 0.01; Figure 1C). These results indicated that HCC1937 cells were more sensitive to BMN 673. However, BMN 673 was cytotoxic for MDA-MB-231 cells for 12 days treatment. Following treatment of 12 days with 1 nM BMN 673, MCF-10A cells remained nearly 55.0% viable.
Apoptotic Effects of BMN 673 on TNBC
To examine the apoptotic effects of BMN 673, HCC1937, MDA-MB-231, and MCF-10A cells were stained with annexin V and 7-AAD. As shown in Figure 2, BMN 673 induced apoptosis in a dose and time-dependent manner. After treatment with 10 nM BMN 673 for 6 days, the late apoptotic rates were 30.81%, 4.86%, and 17.06% in HCC1937, MDA-MB-231, and MCF-10A cells, respectively (P less than 0.01, Figure 2A). However, the ratio of late apoptotic cells was 79.65%, 46.89%, and 26.11% at 10 nM BMN 673 for 12 days in HCC1937, MDA-MB-231, and MCF-10A cells, respectively (P less than 0.01; Figure 2B). These results demonstrated that BMN 673 exerted apoptotic effects, particularly in late stage apoptosis in cells. However, BMN 673 had remarkably more apoptotic effects on HCC1937 cells than MDA-MB-231 cells.
The Effects of BMN 673 on Cell-Cycle Arrest
Next, we investigated the cell cycle distribution in BMN 673-treated HCC1937, MDA-MB-231, and MCF-10A cells and the obtained results were demonstrated in Figure 3. 10 nM BMN 673 treatment caused a significant increase in the population of cells in G2/M phase of the cell cycle from control (40.4%) to treated HCC1937 cells (44.5%), from control (31.5%) to treated MDA-MB-231 cells (41.9%) and from control (22.3%) and to treated MCF-10A cells (31.2%) for 6 days (P less than 0.01; Figure 3A). On the other hand, HCC1937, MDA-MB-231, and MCF-10A cells treated with 10 nM BMN 673 for 12 days indicated a remarkable G2/M arrest (49.3%, 45.6%, and 40.0%, respectively) compared with 36.4%, 32.5%, and 18.5% in the control, respectively (P less than 0.01; Figure 3B). Therefore, our data demonstrated that BMN 673 induced cell-cycle arrest at the G2/M phase.
Multicaspase Activation
Multicaspase activity was performed in BMN 673-treated HCC1937, MDA-MB-231, and MCF-10A cells to further confirm the induction of apoptosis. With 12 days treatment of 10 nM BMN 673, the proportion of caspase positive/dead cells considerably increased from 0.0%, 3.22%, and 7.64% in control cells to 67.73%, 39.57%, and 42.11% in HCC1937, MDA-MB-231, and MCF-10A cells, respectively (P less than 0.01; Figure 4). Therefore, our results indicated that BMN 673 significantly increased multicaspase activity.
Cell Morphology
AO/Et-Br staining was performed in HCC1937, MDA-MB-231, and MCF-10A cells treated with 10 nM BMN 673 (Figure 4). In HCC1937 and MDA-MB-231 cells, chromatin condensation and/or fragmentation and many vacuoles (vacuolization) were observed in response to 10 nM BMN 673 treatment. Additionally, some necrotic cells were observed in MDA-MB-231 cells at 0.01 nM BMN 673 for 12 days. In MCF-10A cells, the general morphology was similar to the control group after treatment with 0.01 nM BMN 673. However, chromatin condensation and rounded cell morphology were also observed at 10 nM BMN 673 in MCF-10A cells. Therefore, BMN 673 especially induced late apoptosis in TNBC cell lines. However, BMN 673 caused more damage in HCC1937 cells.
Apoptotic Gene Expression Profile
The mRNA levels of Bax, Bcl-2, CCND1, RB1, and CDKN1A in HCC1937, MDA-MB-231, and MCF-10A cells were analyzed by quantitative reverse-transcription PCR and the results were presented in Figure 5. After 6 and 12 days exposure to 10 nM BMN 673, the mRNA expression levels of Bax, Bcl-2, CCND1, RB1, and CDKN1A in HCC1937 cells were significantly elevated compared with the control cells (Figure 5A). The mRNA expression of Bax, Bcl-2, CCND1, RB1, and CDKN1A was significantly nearly 6.9-fold (P = 0.000), 2.3-fold (P = 0.021), 9.0-fold (P = 0.000), 5.1-fold (P = 0.004), and 8.3-fold (P = 0.009) higher, respectively in HCC1937 cells following treatment with 10 nM BMN 673 for 12 days (Figure 5A). Only the Bcl-2 (2.9-fold; P = 0.004) and CDKN1A (1.4-fold; P = 0.011) expression were upregulated in MDA-MB-231 cells treated with 10 nM BMN 673 for 6 days. However, Bax, Bcl-2, CCND1, RB1, and CDKN1A mRNA levels were considerably upregulated about 4.0-fold (P = 0.000), 6.6-fold (P = 0.005), 1.9-fold (P = 0.000), 2.1-fold (P = 0.000), and 5.6-fold (P = 0.000), respectively, in MDA-MB-231 cells following treatment with at a dose of 10 nM BMN 673 for 12 days (Figure 5B). In addition, treatment with 10 nM BMN 673 increased the Bax, Bcl-2, CCND1, RB1, and CDKN1A expression levels nearly 2.8-fold (P = 0.000), 4.0-fold (P = 0.000), 4.9-fold (P = 0.136), 2.0-fold (P = 0.000), and 5.9-fold (P = 0.000), respectively, in MCF-10A cells due to induction of apoptosis (Figure 5C). Thus, BMN 673 lead to apoptotic cell death via upregulation of related proteins that induce apoptosis, including Bax and CDKN1A.
Discussion
In the present study, we investigated the potential therapeutic effects of BMN 673 on TNBC cell lines with different genetic backgrounds. Our findings indicated that BMN 673 significantly reduced the cell viability of HCC1937 and MDA-MB-231 via apoptotic cell death. However, BRCA1 mutant HCC1937 cells were more sensitive to BMN 673-induced damage than MDA-MB-231 cells. Additionally, we demonstrated that BMN 673 was cytotoxic for MCF-10A cells especially after 12 days exposure to 1 and 10 nM BMN 673. Thus, higher concentrations and/or longer exposure times might be necessary to achieve better therapeutic response to BMN 673 in MDA-MB-231 cells. On the other hand, treatment of TNBC cells with low nanomolar concentration of BMN 673 are more potent and effective than other PARP inhibitors in BRCA- and/or HR-deficient tumors.
In the current study, we found that BMN 673-induced apoptotic cell death via multicaspase activation in TNBC cell lines with different genetic profile. Additionally, we determined high expression of Bax gene and low expression of Bcl-2 gene in the HCC1937 cells incubated with BMN 673. However, MDA-MB-231 cells expressed significantly higher levels of Bcl-2 than Bax after treatment with 10 nM BMN 673 for 12 days. Interaction between Bcl-2 and Bax is critical point in the regulation of cytochrome c release from mitochondria and induction of the intrinsic apoptosis pathway. Overexpression of Bcl-2 has been tightly linked to tumor development and apoptosis resistance in some tumors. However, recent studies have suggested that Bcl-2 may function as a switch between an antiapoptotic protein and a proapoptotic molecule under certain circumstances (caspase cleavage and downregulating Bcl-xL). Therefore, the role of Bcl-2 in BMN 673-induced apoptosis still remains to be clarified. In addition, further research will be required to define changes in apoptosis-related gene expression profile.
In basal-like and TNBC tumors, mutations of BRCA, p53, ATM, CDKN2A, and PIK3CA, loss of PTEN, RB1, and BRCA1, amplification of cyclin E1 and increased expression of MYC and HIF1-alpha have been reported in the literature. HCC1937 cells and MDA-MB-231 cells carried c.916 C to T and c.839 G to A mutation in p53 gene, respectively. Additionally, MDA-MB-231 cells carried c.1_471del471 mutation in CDKN2A gene. Thus, changes in the expression of other DNA repair and cell-cycle checkpoint genes could also contribute to therapeutic effects of PARP inhibitors in TNBC cells. In this context, further studies are needed to explore association between differential drug responses and genetic profile in TNBC.
G2/M arrest by the PARP inhibitors has linked to activation and/or inhibition signal transduction pathways involving cell-cycle proteins p21 and cyclins and extracellular signal-regulated kinase-dependent kinase cascades. Our findings demonstrated that BMN 673 significantly induced G2/M phase cell-cycle arrest in HCC1937 and MDA-MB-231 cells. However, we found that the extend of G2/M arrest was nearly similar in these cells. Comparison of expression patterns of key cell cycle-regulated genes (CCDN1, RB1, and CDKN1A) in HCC1937 and MDA-MB-231 cells, the CCDN1, RB1, and CDKN1A mRNA levels were higher in HCC1937 cells than MDA-MB-231 cells. Overexpression of CDKN1A (p21) increase G2/M arrest, whereas increased level of CDKN1A induces activation of cyclin D-CDK4 complex and phosphorylation RB1 in G1-phase under conditions of normal cellular proliferation. Thus, a further link between cyclins, cell cycle-specific expression and check-points, and efficacy of BMN 673 still need to be fully elucidated.
Conclusion
In the current study, for the first time, it was seen that BMN 673 showed greater potency in both BRCA1 mutant and wild-type TNBC cells. However, BMN 673 was more effective in BRCA1 mutant HCC1937 cells than MDA-MB-231 cells. These differences may result from changes in the transcription of apoptosis-related gene expression and also genetic background of TNBC cell line. However, this study should be improved by elucidation of the apoptotic pathways, regulation of mitochondrial function, and epigenetic mechanism and also in vivo studies.
